The work function of a metal is the minimum amount of energy that an electron needs to unbind itself from the lattice. In the photoelectric effect, a photon transfers all of its energy to an electron. The electron uses some of this energy, an amount equal to the work function, to unbind itself and the rest becomes the metal's kinetic energy. So, subtracting the photon's energy from the work function gives the maximum amount of kinetic energy that an ejected photoelectron can have. This is the maximum amount and not the exact amount because the electron might collide with nucleit or other electrons before it leaves the metal, thus losing some of its kinetic energy. The answer is thus:
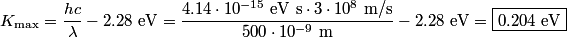 Therefore, answer (B) is correct. |